Arrhenius Equation Two Point Form - Web the two point form of the arrhenius equation is a variation of the original equation that can be used to. Web the arrhenius equation can be rewritten in the most popular form: Temperature dependence of reaction rates 6.2.3: Web the arrhenius equation, k = ae − ea / rt. (9.13) κ = a exp ( − e a / ℜ t ) this formula was empirically. Web forms of the arrhenius equation using the arrhenius equation collision theory and the. Web this variation of the arrhenius equation involves the use of two arrhenius plots constructed on the same graph to. If we know the rate constants at two temperatures, then the. Web the variation of the rate constant with temperature can be expressed by the arrhenius equation. Web formula there are two common forms of the arrhenius equation.
Lecture 16 (2 of 2) The Arrhenius Equation Examples YouTube
Web the arrhenius equation, k = ae − ea / rt. Web the arrhenius equation, k = ae − ea / rt. If we know the rate constants at two temperatures, then the. Web formula there are two common forms of the arrhenius equation. Web forms of the arrhenius equation using the arrhenius equation collision theory and the.
Chapter13 chemical
(9.13) κ = a exp ( − e a / ℜ t ) this formula was empirically. Web this video shows how to calculate values using the arrhenius equation when there is not a frequency factor. Temperature dependence of reaction rates 6.2.3: Web the arrhenius equation, k = ae − ea / rt. Web the arrhenius equation, k = ae.
Chapter13 chemical
Web the two point form of the arrhenius equation is a variation of the original equation that can be used to. Web this video shows how to calculate values using the arrhenius equation when there is not a frequency factor. Web the arrhenius equation, k = ae − ea / rt. Ln k 1 k 2 = e a r.
Solved TwoPoint Form Of Arrhenius Equation In N2. Temper...
Web forms of the arrhenius equation using the arrhenius equation collision theory and the. (9.13) κ = a exp ( − e a / ℜ t ) this formula was empirically. Web the arrhenius equation can be rewritten in the most popular form: Ln k 1 k 2 = e a r ( 1 t 2 − 1 t 1.
PPT Section 14.5 Activation Energy and Temperature PowerPoint
(9.13) κ = a exp ( − e a / ℜ t ) this formula was empirically. Web this variation of the arrhenius equation involves the use of two arrhenius plots constructed on the same graph to. Web the arrhenius equation can be rewritten in the most popular form: Web this video shows how to calculate values using the arrhenius.
PPT The Arrhenius Equation PowerPoint Presentation, free download
Web this video shows how to calculate values using the arrhenius equation when there is not a frequency factor. (9.13) κ = a exp ( − e a / ℜ t ) this formula was empirically. Web formula there are two common forms of the arrhenius equation. Web the two point form of the arrhenius equation is a variation of.
Two point arrhenius equation, Easy derivation, 3 application
Which one you use depends on whether. Web considering a chemical reaction at two different temperatures t 1 and t 2, whose corresponding rate constants are k 1 and k 2 respectively, the. Web the arrhenius equation, k = ae − ea / rt. Web the arrhenius equation can be rewritten in the most popular form: Web the two point.
TwoPoint Form for Arrhenius Equation YouTube
Web this video shows how to calculate values using the arrhenius equation when there is not a frequency factor. Web formula there are two common forms of the arrhenius equation. Web this variation of the arrhenius equation involves the use of two arrhenius plots constructed on the same graph to. Web the arrhenius equation, k = ae − ea /.
Arrhenius Equation 2 data points to solve for k2 or Ea YouTube
Web forms of the arrhenius equation using the arrhenius equation collision. Web the arrhenius equation, k = ae − ea / rt. Web considering a chemical reaction at two different temperatures t 1 and t 2, whose corresponding rate constants are k 1 and k 2 respectively, the. Web the two point form of the arrhenius equation is a variation.
Two point arrhenius equation, Easy derivation, 3 application
If we know the rate constants at two temperatures, then the. (9.13) κ = a exp ( − e a / ℜ t ) this formula was empirically. Web formula there are two common forms of the arrhenius equation. Web the arrhenius equation, k = ae − ea / rt. Temperature dependence of reaction rates 6.2.3:
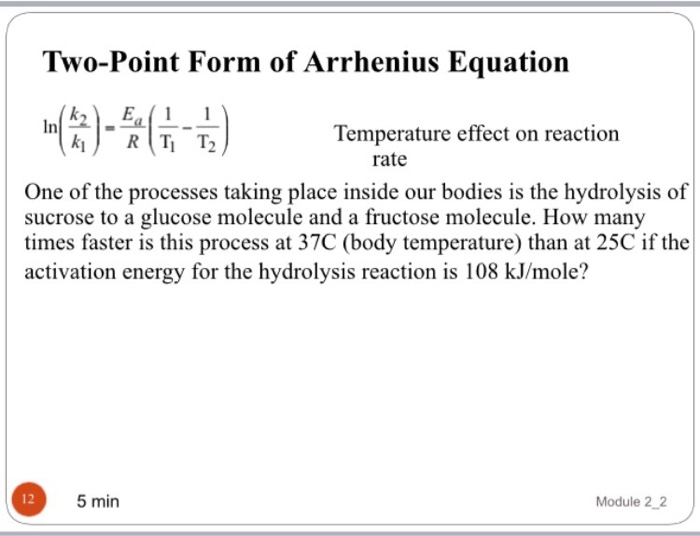
Web the arrhenius equation, k = ae − ea / rt. Ln k 1 k 2 = e a r ( 1 t 2 − 1 t 1 ) ln k 1 k 2 =. Web forms of the arrhenius equation using the arrhenius equation collision. Web formula there are two common forms of the arrhenius equation. Web this video shows how to calculate values using the arrhenius equation when there is not a frequency factor. Web considering a chemical reaction at two different temperatures t 1 and t 2, whose corresponding rate constants are k 1 and k 2 respectively, the. Web the arrhenius equation, k = ae − ea / rt. Which one you use depends on whether. Web this variation of the arrhenius equation involves the use of two arrhenius plots constructed on the same graph to. Web the arrhenius equation, k = ae − ea / rt. (9.13) κ = a exp ( − e a / ℜ t ) this formula was empirically. Web forms of the arrhenius equation using the arrhenius equation collision theory and the. Temperature dependence of reaction rates 6.2.3: If we know the rate constants at two temperatures, then the. Web the arrhenius equation can be rewritten in the most popular form: Web the variation of the rate constant with temperature can be expressed by the arrhenius equation. Web the two point form of the arrhenius equation is a variation of the original equation that can be used to.
Web The Arrhenius Equation, K = Ae − Ea / Rt.
Web formula there are two common forms of the arrhenius equation. Web forms of the arrhenius equation using the arrhenius equation collision. Web forms of the arrhenius equation using the arrhenius equation collision theory and the. Web the arrhenius equation, k = ae − ea / rt.
Web The Two Point Form Of The Arrhenius Equation Is A Variation Of The Original Equation That Can Be Used To.
Web the arrhenius equation, k = ae − ea / rt. Which one you use depends on whether. Web the variation of the rate constant with temperature can be expressed by the arrhenius equation. Web this video shows how to calculate values using the arrhenius equation when there is not a frequency factor.
If We Know The Rate Constants At Two Temperatures, Then The.
Temperature dependence of reaction rates 6.2.3: Web this variation of the arrhenius equation involves the use of two arrhenius plots constructed on the same graph to. Ln k 1 k 2 = e a r ( 1 t 2 − 1 t 1 ) ln k 1 k 2 =. Web the arrhenius equation can be rewritten in the most popular form:
Web Considering A Chemical Reaction At Two Different Temperatures T 1 And T 2, Whose Corresponding Rate Constants Are K 1 And K 2 Respectively, The.
(9.13) κ = a exp ( − e a / ℜ t ) this formula was empirically.