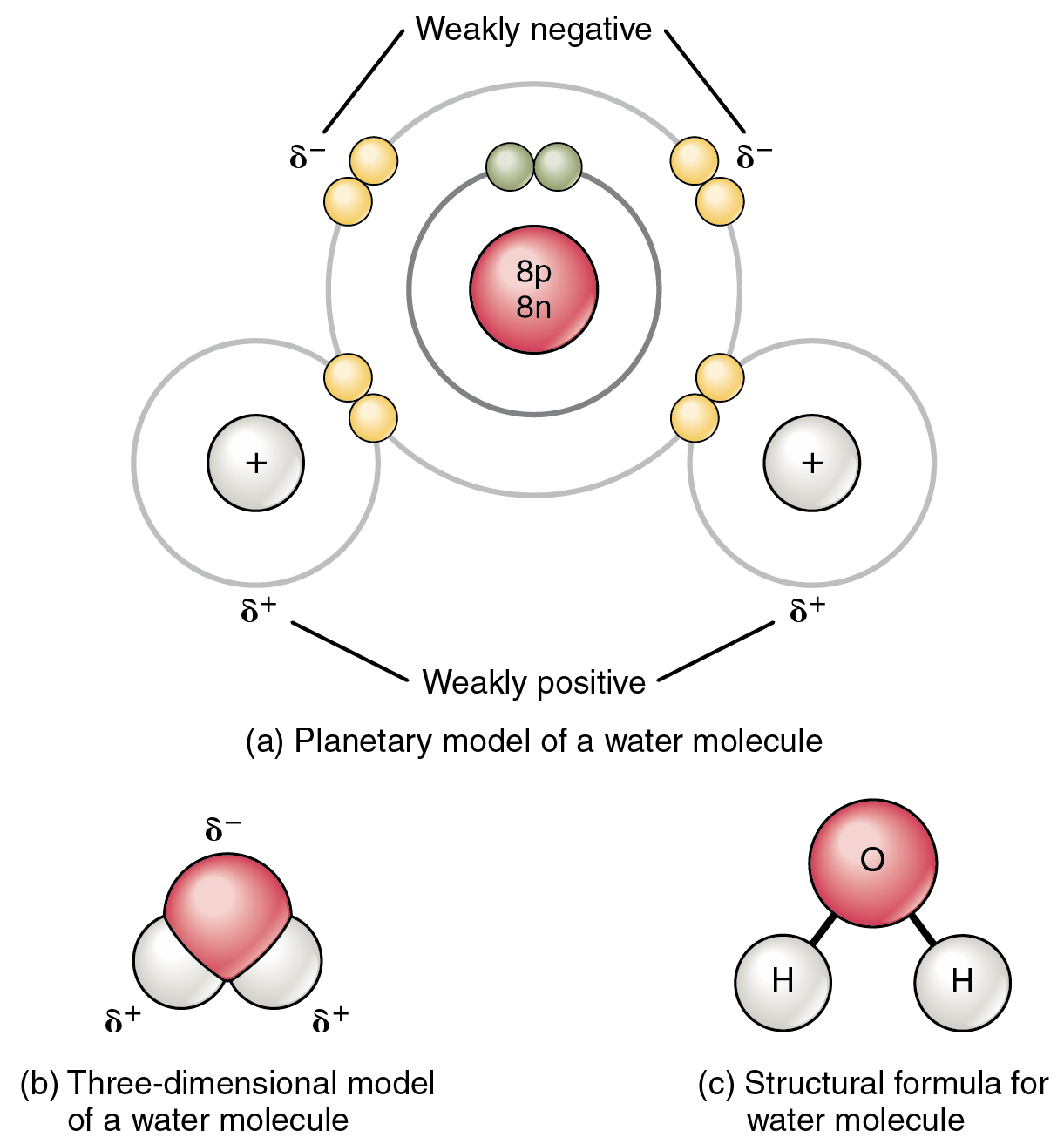
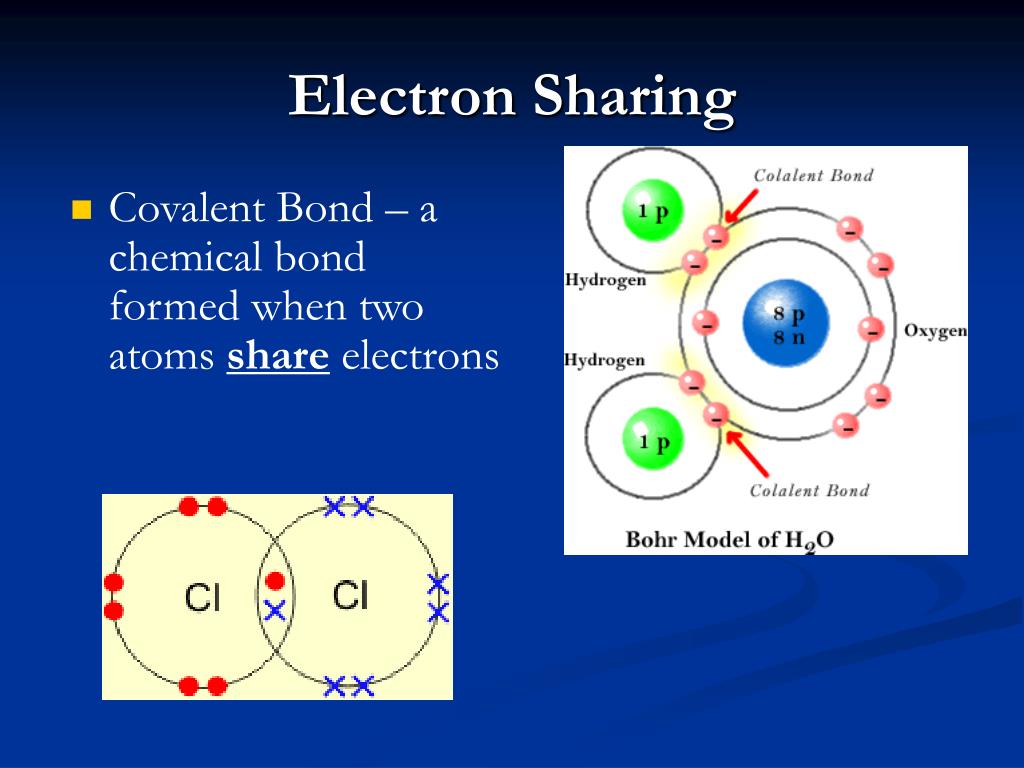
Covalent Bonds Are Formed When Electrons Are - Covalent bonding occurs when pairs of electrons are shared by atoms. Web a chemical bond that forms between nonmetals and/or metalloids that is the result of sharing their valence electrons. Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Let's illustrate how a covalent bond forms. These electron pairs are known as shared pairs. In pure covalent bonds, the electrons are shared. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Atoms will covalently bond with other atoms in order to gain more stability,. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms.
covalent bond Definition, Properties, Examples, & Facts Britannica
Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. In pure covalent bonds, the electrons are shared. Let's illustrate how a covalent bond forms. These electron pairs are known as shared pairs. Covalent bonding occurs when pairs of electrons are shared by atoms.
Attractive Forces and Bonds
Let's illustrate how a covalent bond forms. Atoms will covalently bond with other atoms in order to gain more stability,. These electron pairs are known as shared pairs. Web a chemical bond that forms between nonmetals and/or metalloids that is the result of sharing their valence electrons. Web a covalent bond is a chemical bond that involves the sharing of.
Covalent Bond sharing of electrons between atoms; bonds contain energy
In pure covalent bonds, the electrons are shared. Atoms will covalently bond with other atoms in order to gain more stability,. Covalent bonding occurs when pairs of electrons are shared by atoms. Let's illustrate how a covalent bond forms. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms.
PPT Chapter 5 Atoms & Bonding PowerPoint Presentation, free
Covalent bonding occurs when pairs of electrons are shared by atoms. Atoms will covalently bond with other atoms in order to gain more stability,. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Web a chemical bond that forms between nonmetals and/or metalloids that is the result of sharing their valence.
The top panel in this figure shows two hydrogen atoms sharing two
Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. These electron pairs are known as shared pairs. Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Let's illustrate how a covalent bond forms. Atoms will covalently bond with.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Web a chemical bond that forms between nonmetals and/or metalloids that is the result of sharing their valence electrons. Let's.
Covalent Bonding (Biology) — Definition & Role Expii
Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Let's illustrate how a covalent bond forms. These electron pairs are known as shared pairs. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Covalent bonding occurs when pairs.
Introducing Covalent Bonding Montessori Muddle
Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Atoms will covalently bond with other atoms in order to gain more stability,. Covalent bonding occurs when pairs of electrons are shared by atoms. Let's illustrate how a covalent bond forms. Covalent bonds form when electrons are shared.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
These electron pairs are known as shared pairs. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Let's illustrate how a covalent bond forms. In pure covalent.
Covalent Bond Easy Science Covalent bonding, Easy science, Cool
Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Web a chemical bond that forms between nonmetals and/or metalloids that is the result of sharing their valence electrons. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between.
These electron pairs are known as shared pairs. Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). In pure covalent bonds, the electrons are shared. Covalent bonding occurs when pairs of electrons are shared by atoms. Web a chemical bond that forms between nonmetals and/or metalloids that is the result of sharing their valence electrons. Atoms will covalently bond with other atoms in order to gain more stability,. Let's illustrate how a covalent bond forms. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms.
These Electron Pairs Are Known As Shared Pairs.
Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Let's illustrate how a covalent bond forms. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals).
Covalent Bonding Occurs When Pairs Of Electrons Are Shared By Atoms.
Atoms will covalently bond with other atoms in order to gain more stability,. In pure covalent bonds, the electrons are shared. Web a chemical bond that forms between nonmetals and/or metalloids that is the result of sharing their valence electrons.