The Type Of Bond Formed By Shared Electrons Is - Web the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. The chemical bond that is formed between two combining atoms by mutual sharing of one. Web electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent bond (e.g.,. A covalent bond forms when atoms share electrons. B) nonmetals can share pairs of electrons and form ionic bonds. So those electrons belong to both of those atoms. Web in covalent bonding electrons are shared between two atoms. Ionic bonds require at least one electron donor and one. Web sodium (na) only has one electron in its outer electron shell, so it is easier (more energetically favorable) for sodium to donate that one electron than to find. Web a chemical bond is a force of attraction between atoms or ions.
hillis2e_ch02
Web when a bond is formed by sharing electrons, it's called a covalent bond. Web a chemical bond is a force of attraction between atoms or ions. So those electrons belong to both of those atoms. Web when electrons are shared between two atoms, they make a bond called a covalent bond. Sometimes the electrons in a covalent bond are.
PPT Chapter 5 Atoms & Bonding PowerPoint Presentation, free
Web the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Web electrons shared in pure covalent bonds have an equal probability of being near each nucleus. In the case of cl 2,.
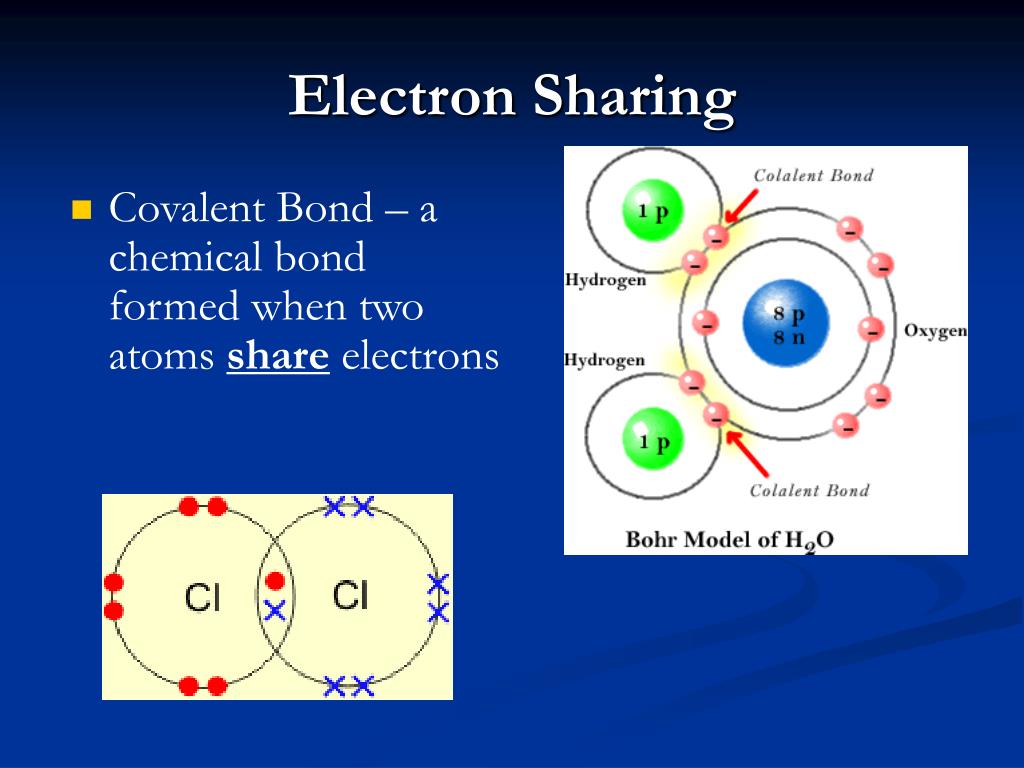
Covalent Bond sharing of electrons between atoms; bonds contain energy
Web in covalent bonding electrons are shared between two atoms. Web when a bond is formed by sharing electrons, it's called a covalent bond. Web the correct option is b. So those electrons belong to both of those atoms. Web when electrons are shared between two atoms, they make a bond called a covalent bond.
Bonding A Level Notes
Web sodium (na) only has one electron in its outer electron shell, so it is easier (more energetically favorable) for sodium to donate that one electron than to find. Web in ionic bonding, atoms transfer electrons to each other. Web electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent bond (e.g.,. Web the correct.
Covalent Bond Easy Science Covalent bonding, Easy science, Cool
Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. A type of chemical bond where two atoms are connected to each other by the sharing of. Web when electrons are shared by two metallic atoms a metallic bond may be formed. Bonds form when atoms share or transfer valence electrons. Web.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Web in a polar covalent bond, the electrons shared by the atoms spend more time closer to one nucleus than to the. Bonds form when atoms share or transfer valence electrons. Web the correct option is b. Web when a bond is formed by sharing electrons, it's called a covalent bond. Web a) metals will only form covalent bonds.
Covalent bonds Learning Lab
Web in covalent bonding electrons are shared between two atoms. When two atoms form a covalent bond, they. Web a chemical bond is a force of attraction between atoms or ions. Web in a polar covalent bond, the electrons shared by the atoms spend more time closer to one nucleus than to the. Web the correct option is b.
Ionic Bond Definition, Types, Properties & Examples
Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Web a chemical bond is a force of attraction between atoms or ions. Web a) metals will only form covalent bonds. When two atoms form a covalent bond, they. The chemical bond that is formed between two combining atoms by mutual sharing.
What Is A Covalent Bond In Chemistry? AA3
Web the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Web the correct option is b. Web a chemical bond is a force of attraction between atoms or ions. Ionic bonds require at least one electron donor and one. Web in ionic bonding, atoms transfer electrons to each other.
The top panel in this figure shows two hydrogen atoms sharing two
Web covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Web when electrons are shared between two atoms, they make a bond called a covalent bond. Web the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. A type of chemical.
Web in ionic bonding, atoms transfer electrons to each other. In the case of cl 2, each atom. When two atoms form a covalent bond, they. Web the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. Web in a polar covalent bond, the electrons shared by the atoms spend more time closer to one nucleus than to the. A type of chemical bond where two atoms are connected to each other by the sharing of. Web 3 rows because they have the same electronegativity, they will share their valence electrons equally. Web when a bond is formed by sharing electrons, it's called a covalent bond. So those electrons belong to both of those atoms. Web sodium (na) only has one electron in its outer electron shell, so it is easier (more energetically favorable) for sodium to donate that one electron than to find. Web the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Web a) metals will only form covalent bonds. Web the correct option is b. In a covalent bond, electrons are. Web when electrons are shared between two atoms, they make a bond called a covalent bond. Bonds form when atoms share or transfer valence electrons. Web electrons shared in pure covalent bonds have an equal probability of being near each nucleus. The chemical bond that is formed between two combining atoms by mutual sharing of one. Web in covalent bonding electrons are shared between two atoms.
A Type Of Chemical Bond Where Two Atoms Are Connected To Each Other By The Sharing Of.
The chemical bond that is formed between two combining atoms by mutual sharing of one. Web the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. When two atoms form a covalent bond, they.
Web Electrons Shared In Pure Covalent Bonds Have An Equal Probability Of Being Near Each Nucleus.
Web when electrons are shared by two metallic atoms a metallic bond may be formed. Ionic bonds require at least one electron donor and one. Bonds form when atoms share or transfer valence electrons. Web when a bond is formed by sharing electrons, it's called a covalent bond.
B) Nonmetals Can Share Pairs Of Electrons And Form Ionic Bonds.
So those electrons belong to both of those atoms. Web a) metals will only form covalent bonds. Web the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. In a covalent bond, electrons are.
Web The Types Of Covalent Bonds Can Be Distinguished By Looking At The Lewis Dot Structure Of The Molecule.
Sometimes the electrons in a covalent bond are shared. Web a chemical bond is a force of attraction between atoms or ions. A covalent bond forms when atoms share electrons. Web electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent bond (e.g.,.