What Ions Are Formed When Alkalis Dissolve In Water - Web alkalisclosealkalisubstance producing more hydroxide ions than hydrogen ions when dissolved in water.form. They break up completely to produce a high. Web when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Web c5.3.4 recall that acids form hydrogen ions when they dissolve in water and solutions of alkalis contain hydroxide ions; Web what type of ions is formed when an acid is dissolved in water? Web they form alkali metal cations and hydroxide ions. Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. This is why water is formed in these. And we represent this by the given reaction: Web when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries.
PPT The Nature of Aqueous Solutions and Molarity and Solution
Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. Web recall that acids form hydrogen ions when they dissolve in water and solutions of alkalis contain hydroxide ions. Alkali turns universal indicator blue or violet, and litmus blue. They break up completely to produce a.
PPT Mrs Teocc PowerPoint Presentation, free download ID1776788
Web soluble in water are called alkalis and they dissolve in water to form alkaline solutions. Web they form alkali metal cations and hydroxide ions. Copper oxide is a base, but it. And we represent this by the given reaction: Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h.
PPT Unit 6 TOXINS Solutions & PowerPoint Presentation ID
Web they form alkali metal cations and hydroxide ions. Cbse english medium class 10. Alkali turns universal indicator blue or violet, and litmus blue. Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. Copper oxide is a base, but it.
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free
Copper oxide is a base, but it. And we represent this by the given reaction: Web part of science chemistry what are acids? M (s) + h_2o (l) rarr m^. Web c5.3.4 recall that acids form hydrogen ions when they dissolve in water and solutions of alkalis contain hydroxide ions;
PPT Chapter 3 Water and Life PowerPoint Presentation, free download
And we represent this by the given reaction: Web part of science chemistry what are acids? Web when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Water is needed for any alkali to become truly basic, because only then can. Alkali metals a common characteristic of most alkali metals is their ability to displace.
PPT Acids and Alkalis PowerPoint Presentation, free download ID1994710
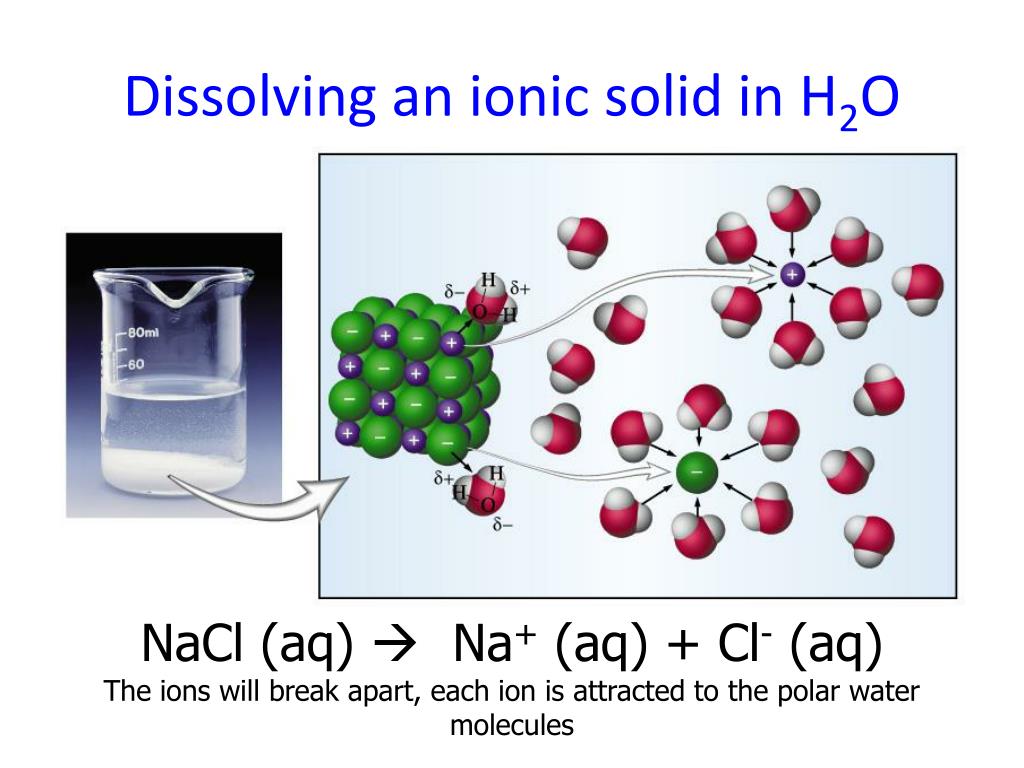
Web when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Web when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. Web soluble in water are called alkalis and they dissolve in water to form alkaline solutions. Water is.
PPT UNIT 5 PowerPoint Presentation, free download ID6635190
Web part of science chemistry what are acids? Cbse english medium class 10. This is why water is formed in these. Water is needed for any alkali to become truly basic, because only then can. Web when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because.
Role of Water to Show Properties of Acids SPM Chemistry
Copper oxide is a base, but it. Web alkalisclosealkalisubstance producing more hydroxide ions than hydrogen ions when dissolved in water.form. This is why water is formed in these. Web when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. And we represent this by the given reaction:
Sodium hydroxide (NaOH) is classified as a strong base. For every mole
Acids and alkalis are common in daily life. Copper oxide is a base, but it. Web alkalisclosealkalisubstance producing more hydroxide ions than hydrogen ions when dissolved in water.form. And we represent this by the given reaction: They are found in the home, in our bodies,.
What is an Alkali and Alkaline solution?
Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. M (s) + h_2o (l) rarr m^. They are found in the home, in our bodies,. Web recall that acids form hydrogen ions when they dissolve in water and solutions of alkalis contain hydroxide ions. This.
Web recall that acids form hydrogen ions when they dissolve in water and solutions of alkalis contain hydroxide ions. Web alkalisclosealkalisubstance producing more hydroxide ions than hydrogen ions when dissolved in water.form. Web when an alkali metal is dissolved in liquid ammonia, it results in the formation of a deep blue coloured solution. Alkali turns universal indicator blue or violet, and litmus blue. Web they form alkali metal cations and hydroxide ions. This is why water is formed in these. Web do all alkalis dissolved in water? Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. Web c5.3.4 recall that acids form hydrogen ions when they dissolve in water and solutions of alkalis contain hydroxide ions; Alkali metals a common characteristic of most alkali metals is their ability to displace h 2 (g) from water. Web soluble in water are called alkalis and they dissolve in water to form alkaline solutions. Copper oxide is a base, but it. Cbse english medium class 10. Web part of science chemistry what are acids? M (s) + h_2o (l) rarr m^. Acids and alkalis are common in daily life. Web key fact (higher tier) strong alkalis completely ionise in water. And we represent this by the given reaction: Water is needed for any alkali to become truly basic, because only then can. Web when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules.
Acids And Alkalis Are Common In Daily Life.
Web soluble in water are called alkalis and they dissolve in water to form alkaline solutions. Web alkalisclosealkalisubstance producing more hydroxide ions than hydrogen ions when dissolved in water.form. M (s) + h_2o (l) rarr m^. Alkali turns universal indicator blue or violet, and litmus blue.
They Break Up Completely To Produce A High.
Web when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. Web when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries. Cbse english medium class 10. Alkali metals a common characteristic of most alkali metals is their ability to displace h 2 (g) from water.
This Is Why Water Is Formed In These.
Copper oxide is a base, but it. Web when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. And we represent this by the given reaction: Web c5.3.4 recall that acids form hydrogen ions when they dissolve in water and solutions of alkalis contain hydroxide ions;
Web A Metal Ion In Aqueous Solution Or Aqua Ion Is A Cation, Dissolved In Water, Of Chemical Formula [M(H 2 O) N] Z+.
Web when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Water is needed for any alkali to become truly basic, because only then can. Web key fact (higher tier) strong alkalis completely ionise in water. Web they form alkali metal cations and hydroxide ions.