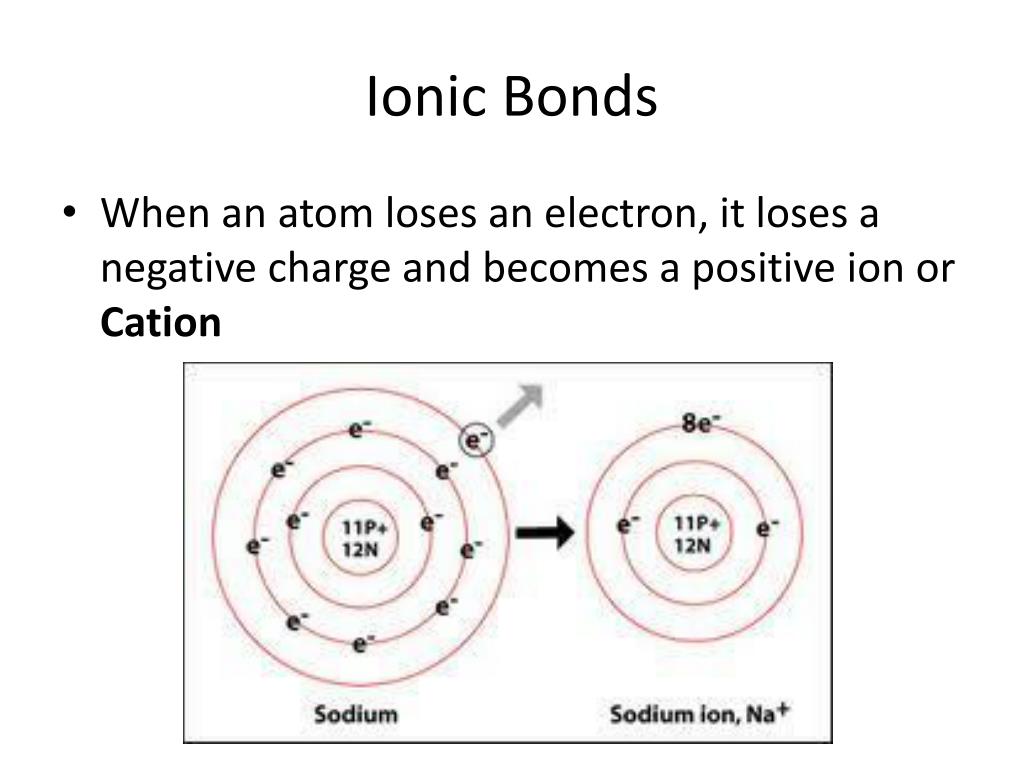
What Type Of Ion Forms When An Atom Loses Electrons - Web when an atom loses electrons, it forms a positive ion, also known as a cation. In a neutral atom, the numbers of protons and. This is because the atom has more protons (positively charged particles) than. Web what type of ion forms when an atom loses electrons? Web when an atom loses one or more electrons, it becomes a positively charged ion, called a cation. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5.
PPT Ionic and Covalent Bonds PowerPoint Presentation, free download
This is because the atom has more protons (positively charged particles) than. Web when an atom loses electrons, it forms a positive ion, also known as a cation. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Web atoms that lose electrons acquire a positive charge as.
PPT Ion Formation PowerPoint Presentation, free download ID2508414
Web what type of ion forms when an atom loses electrons? Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges. In a neutral atom, the numbers of protons and. Web when an atom loses electrons, it forms a positive ion, also known as.
Ionic Bond Definition, Types, Properties & Examples
Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. This is because the atom has more protons (positively charged particles) than. Web when an atom loses electrons, it forms a positive ion, also known as a cation. Web when an atom loses one or more electrons, it.
Ionic Bond Definition, Types, Properties & Examples
In a neutral atom, the numbers of protons and. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. This is because the atom has more protons (positively charged particles) than. Web what type of ion forms when an atom loses electrons? Web atoms that lose electrons acquire.
Formation of Ions and Ionic Compounds Good Science
Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges. Web when an atom loses electrons, it forms a positive ion, also.
Cations and Anions Electrons Gain Lose Stock Vector Illustration of
Web when an atom loses electrons, it forms a positive ion, also known as a cation. Web what type of ion forms when an atom loses electrons? Web when an atom loses one or more electrons, it becomes a positively charged ion, called a cation. In a neutral atom, the numbers of protons and. This is because the atom has.
Ionic Bond Definition, Types, Properties & Examples
Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. In a neutral atom, the numbers of protons and. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges. This is because.
PPT How do atoms form ions? PowerPoint Presentation ID7021047
Web what type of ion forms when an atom loses electrons? In a neutral atom, the numbers of protons and. Web when an atom loses one or more electrons, it becomes a positively charged ion, called a cation. Web when an atom loses electrons, it forms a positive ion, also known as a cation. Web during the formation of some.
Formation of Ion SPM Chemistry
In a neutral atom, the numbers of protons and. Web when an atom loses one or more electrons, it becomes a positively charged ion, called a cation. Web what type of ion forms when an atom loses electrons? Web when an atom loses electrons, it forms a positive ion, also known as a cation. Web during the formation of some.
PPT How do atoms form ions? PowerPoint Presentation, free download
Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. This is because the atom has more protons (positively charged particles) than. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges..
This is because the atom has more protons (positively charged particles) than. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Web what type of ion forms when an atom loses electrons? In a neutral atom, the numbers of protons and. Web when an atom loses electrons, it forms a positive ion, also known as a cation. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges. Web when an atom loses one or more electrons, it becomes a positively charged ion, called a cation.
Web When An Atom Loses One Or More Electrons, It Becomes A Positively Charged Ion, Called A Cation.
Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges. Web when an atom loses electrons, it forms a positive ion, also known as a cation. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. This is because the atom has more protons (positively charged particles) than.
In A Neutral Atom, The Numbers Of Protons And.
Web what type of ion forms when an atom loses electrons?